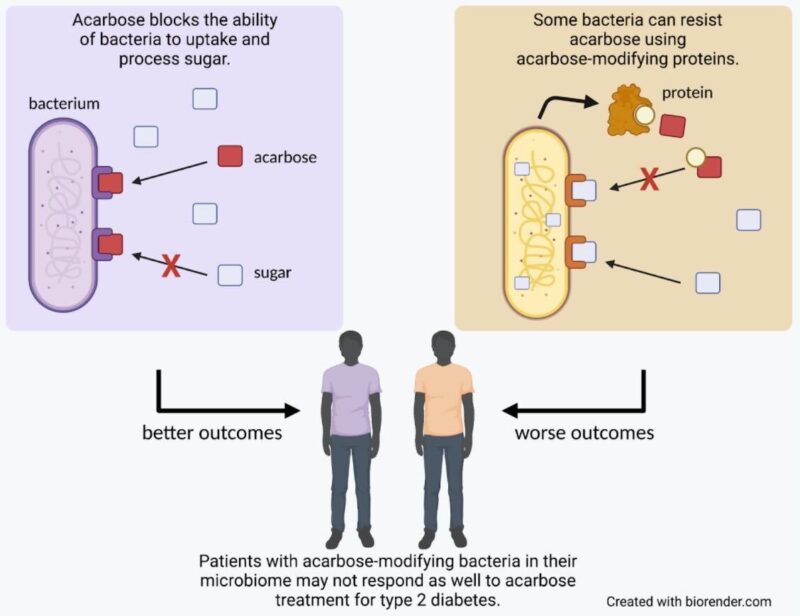
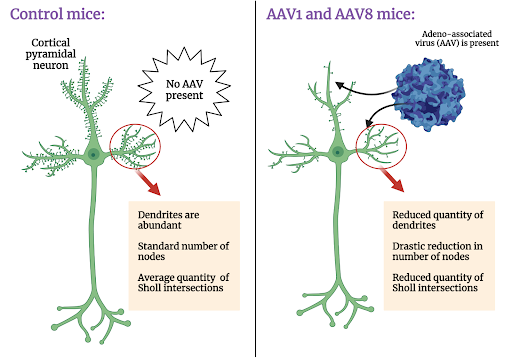
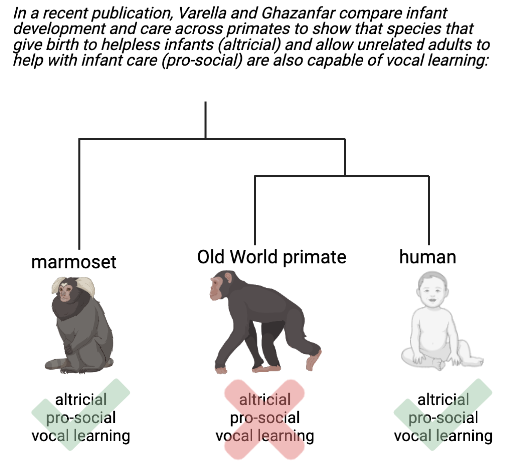
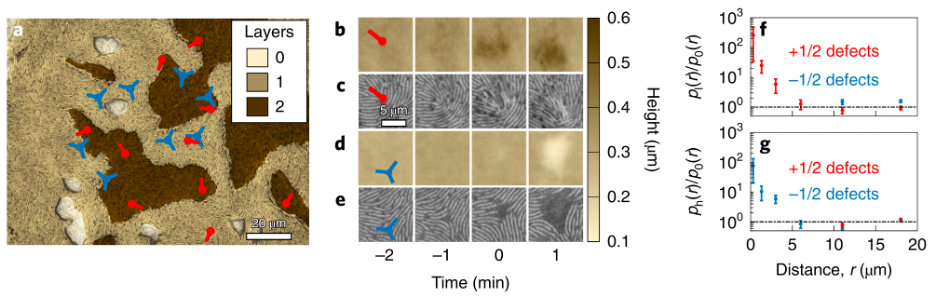
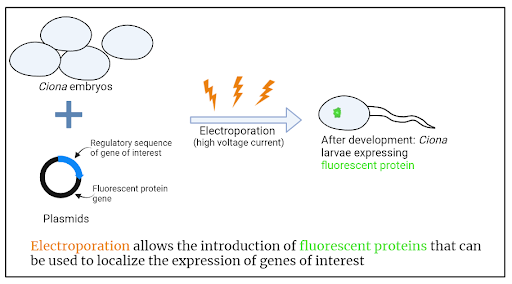
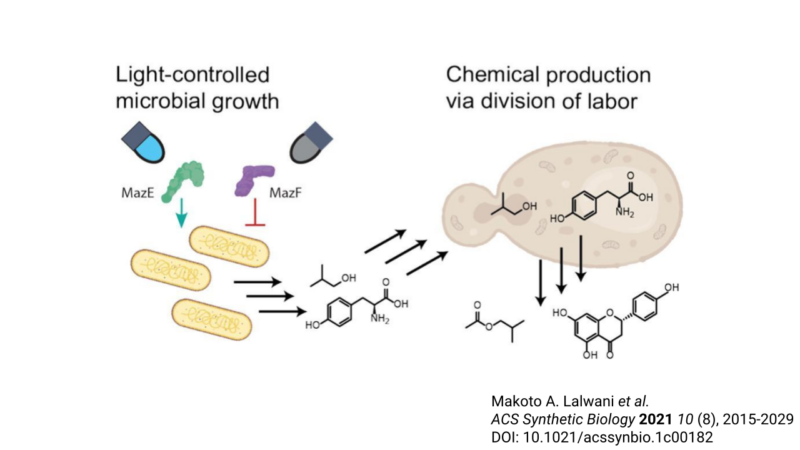
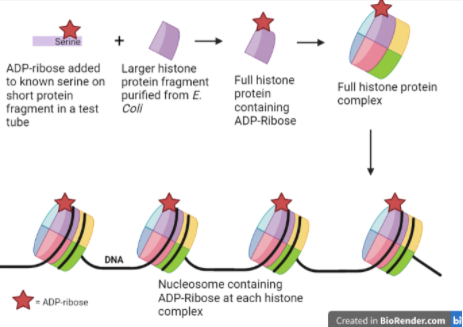
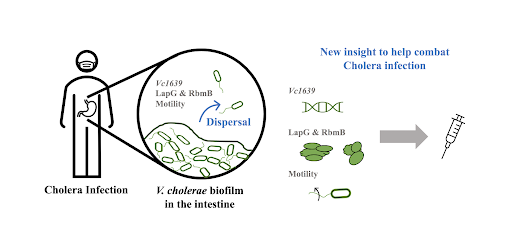
Review written by Jarome Ali (EEB, G5)
On the savannas of Kenya, a battle has been waged for centuries. The landscape hints at how this battle has shaped the entire ecosystem, but it must be viewed from far above. From just a few meters above ground level, the telltale signs are still invisible. However, from the vantage point offered by drone photography or satellite imagery, a clear pattern emerges. Patches of vegetation are spotted across the savanna, in a regular hexagonal layout. This kind of order in the natural world fascinates biologists and begs for an explanation. Researchers at Princeton have been investigating how warring termite colonies (or insect colonies in general), and the underlying resource distribution can drive the emergence of order in the savanna landscape.
Continue reading