Review written by Olivia Duddy (MOL, G5)
Microbes are powerful tools in the biotechnology industry. Like microscopic factories, microbes are employed to manufacture a diversity of chemical compounds, such as industrial chemicals, food products, drugs, and other biotechnology molecules, on a large scale. Given the ease of genetic engineering in microbes like Escherichia coli and Saccharomyces cerevisiae, scientists and metabolic engineers alike tinker with their metabolic capacities, or even completely rewire them, to yield high concentrations of a specific product [1]. Metabolic engineers aim to maximize the efficiency of these biosynthetic processes. High efficiency, in turn, delivers biomolecules that are more readily available and at a lower cost. Metabolic engineering applications also can be more sustainable or environmentally friendly than traditional chemical synthesis approaches [1,2]. Recently, a team of researchers in the Avalos lab, led by former Ph.D. candidate Makoto Lalwani (now postdoctoral researcher at the Wyss Institute), added an additional layer of genetic engineering to this process: They are using light as a strategy to advance biomolecule production [3].
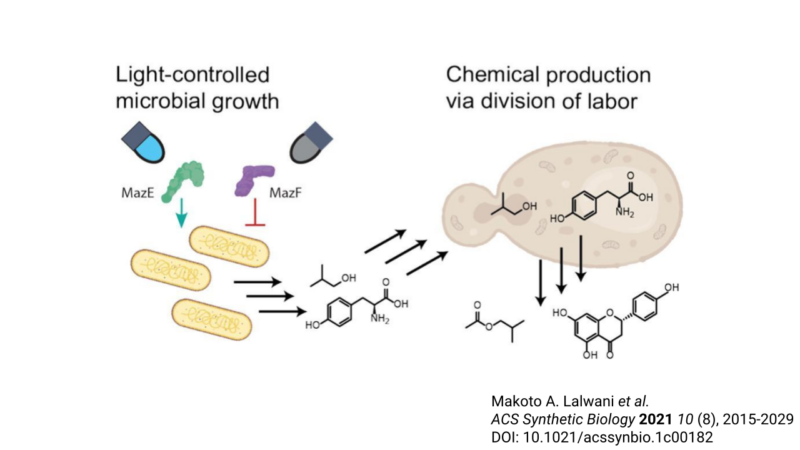
Metabolic engineers often employ a consortium, or group, of microbes to work together to produce a molecule [3]. It is a division-of-labor strategy. Each microbe is responsible for a particular set of steps, called submodules, in the fermentation, or metabolism, of chemicals. While this co-fermentation approach can improve production efficiencies by reducing the burden on a single microbe, it introduces another problem: The fastest-growing microbe tends to outcompete the others. Consequently, the variability in growth rate of each member creates imbalances in the abundances of biosynthetic intermediates, potentially compromising the efficiency of co-culture fermentations. Ideally, metabolic engineers would be able to easily control the abundance of each consortium member throughout the fermentation process to optimize the biosynthesis of a product. However, no strategy has proven capable of robustly addressing this problem. This is where the Avalos group stepped in.
Here, the researchers demonstrated that the growth of E. coli can be finely tuned mid-fermentation using a light-controlled genetic switch, a type of optogenetic regulation. Optogenetics describes the use of light and genetic engineering to precisely control cellular behaviors. Their genetic tool, which they name OptoTA, prevents microbial growth in the dark while allowing growth in blue light. Specifically, the system uses a light-sensitive regulator to control the production of a well-characterized bacterial toxin-antitoxin system. A toxin-antitoxin system is a set of at least two genes that together encode a poison (“toxin”) and antidote (“antitoxin”). In the dark, the toxin MazF is made and E. coli cannot grow. By contrast, in blue light, the antitoxin MazE is made, and MazE neutralizes the toxicity of MazF. Further expression of MazF is also repressed under blue light conditions. As a result, E. coli can grow.
Optogenetics is increasingly employed in a broad diversity of applications given its many advantages. For instance, light is tunable, meaning that the intensity and duration of its exposure can be precisely controlled. It is also reversible: Light can be turned on and off, whereas chemical inducers can be added, but not taken away. Indeed, the researchers demonstrated that they could turn on, or off, the growth of E. coli harboring the OptoTA system. Similarly, by varying the duration of light exposure (i.e., the “duty cycle”), the researchers could finely tune growth rate, a crucial step forward in the dynamic control of fermentation.
The researchers next used OptoTA to control E. coli growth in co-culture with another microbe. For this, they chose S. cerevisiae as its partner. The rate of growth of E. coli is faster than that of S. cerevisiae. Thus, under normal conditions, E. coli will outcompete in co-culture even in cases where S. cerevisiae is inoculated at higher starting concentrations. Indeed, by applying OptoTA, the researchers were capable of balancing the species populations throughout growth.
Finally, the researchers put the OptoTA-controlled co-fermentation system to the test in the production of a biomolecule called isobutyl acetate, a chemical solvent, fragrance, and potential biofuel [3]. Its synthesis requires (1) the production and export of the precursory molecule isobutanol, and (2) combination with another molecule called acetyl-CoA to yield isobutyl acetate. The work required for each of these two submodules were divided between E. coli and S. cerevisiae. Consistent with their prediction, they discovered that finely-tuned light-controlled co-fermentation significantly improved production of isobutyl acetate. Similarly, the researchers also tested their OptoTA system in the production of the natural product naringenin. Naringenin is a key precursor to a class of molecules called flavonoids, which possess promising therapeutic potential [3,4]. Like the case of isobutyl acetate, application of OptoTA to control the fermentation of microbial consortia increased production of naringenin, demonstrating that optogenetics can be employed to improve efficiencies of numerous metabolic pathways.
This work serves as proof-of-principle that light-controlled microbial growth could significantly enhance the production of key biomolecules. In particular, the engineering strategy could be crucial to implementing the widespread use of engineered microbial consortia in chemical production of many pharmaceuticals, supplements, and biofuels [3]. For example, sustainable production of biofuels could replace or alleviate dependence on fossil fuels [1,2]. Natural microbial consortia are already crucial to industries like the dairy industry [3], notably in the production of cheese and yogurt. Despite the proven benefits of distributing metabolic roles among different members, use of microbial consortia has not been widely adopted for metabolic engineering given the challenges in maintaining a stable microbial community once fermentation begins [3]. Thus, optogenetic control could unlock the full capacity of microbial consortia in the biotechnology industry.
Lead author on the work, Dr. Makoto Lalwani, remarks: “I'd like to thank Drs. Tina DeCoste and Katherine Rittenbach of the Flow Cytometry core facility for helping to implement a cell counting method that distinguishes bacteria and yeast populations to characterize the OptoTA circuit.”
The original article described here was published in American Chemical Society Synthetic Biology on August 5, 2021. Please follow this link to view the full version.
Literature Cited:
- Physiological limitations and opportunities in microbial metabolic engineering. López JM, Duran L, & Avalos JL. Nat Rev Microbiol. 2021 Aug 2; doi: 10.1038/s41579-021-00600-0
- Metabolic engineering strategies toward production of biofuels. Choi KR, Jiao S, & Lee SY. Curr Opin Chem Biol. 2020 Dec; 59:1-14.
- Optogenetic Control of Microbial Consortia Populations for Chemical Production. Lalwani MA, Kawabe H, Mays RL, Hoffman SM, & Avalos JL. ACS Synth Biol. 2021 Aug 20; 10(8): 2015-2029.
- De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Koopman F, Beekwilder J, Crimi B, van Houwelingen A, et al. Microb Cell Fact. 2012 Dec 8; 11:155.