Review written by Alexandra Libby (PNI)
Cell division is one of the most important and well-studied biological processes. Organisms generate new cells in order to grow and reproduce (Figure 1); the types of cell division responsible for each of these goals are called mitosis and meiosis, respectively. Like many biological processes, cell division involves a well-timed, complex coordination of proteins and cellular machinery. Disrupted division can lead to a multitude of problems including genetic mutations, cell death, and cancer (Zhivotovsky and Orrenius, 2010).
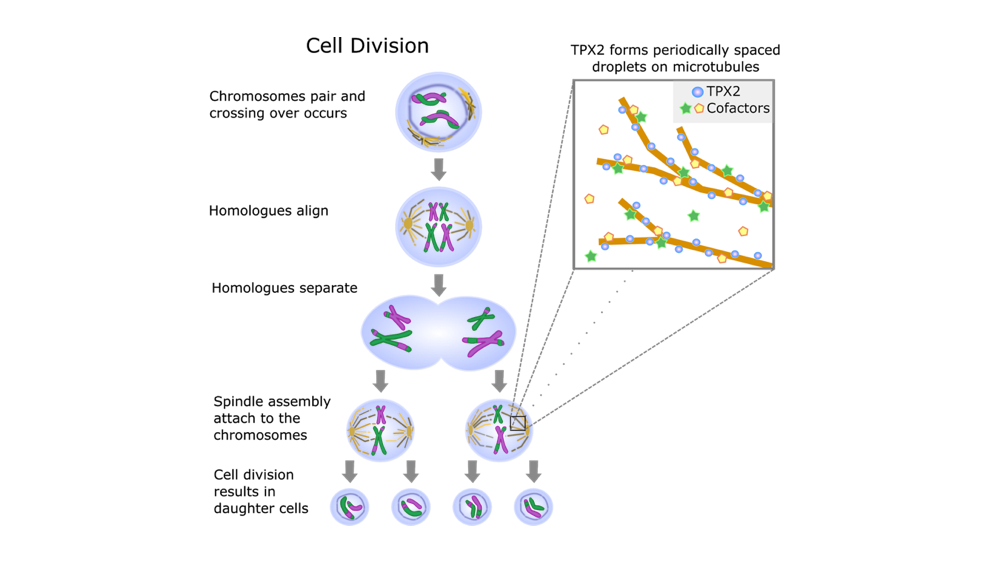
A key step in cell division is the separation of the cell’s genetic material or DNA. Here, the chromosomes (i.e., long chains of DNA) must be divided and carried to the opposite side of the cell. This process is controlled by hollow tubes called microtubules. Microtubules aid cell division by growing and branching to form a “spindle”, a molecular machine that grabs and pulls apart chromosomes. While previous work has implicated microtubules in cell division, the biophysical processes promoting their growth and branching is not well understood (Petry, 2016). Many different molecules are required for fast, precise spindle assembly. Researchers at Princeton are taking an interdisciplinary approach, combining biology with biophysical modeling to better understand how microtubules work during cell division.
The protein TPX2 is a key mediator of microtubule generation. Multiple studies across a variety of species have shown that depleting TPX2 causes defects in spindle assembly. Furthermore, knocking out TPX2 abolished the creation of microtubules (Walczak and Heald, 2008). Importantly, TPX2 initiates the start of microtubule branching nucleation, a process by which new microtubules grow off the sides of preexisting ones (King and Petry, 2020). Continuing on this work in the Petry lab, Setru and Gouveia, in collaboration with the Shaevitz and Stone labs, studied the involvement of TPX2 in microtubule branching nucleation (Setru et al., 2020). For this, they used an in vitro preparation combining purified proteins involved in cell division. This is not an easy process to study! Microtubule polymerization (i.e., the creation of a long chain of identical molecules) occurs on the scale of 1-10s of nanometers, which are one billionth of a meter, smaller than cells and even viruses. Therefore, cutting edge recording techniques are required to observe and perturb these processes. In order to capture these microscopic processes, Setru et al. used electron microscopy (EM), which shoots electrons to capture a high resolution image of a biological sample. To study the dynamics of microtubule formation, they used atomic force microscopy, which maps the surface topography over time, in a process akin to reading braille. Using these techniques, they found that the protein TPX2 coats the microtubule and then forms droplets along it. New microtubule branches grow from these droplets. Because TPX2 does not mix well with water, it separates from the cell’s cytoplasm to form droplets. Importantly, they found that the formation of droplets along the microtubules can be explained by the Rayleigh-Plateau instability, a fluid dynamic model that governs liquid-liquid interactions and droplet formation.
Imagine turning a faucet on low and the stream of water coming out in discrete droplets. The same law can also explain why the oil separates out of your salad dressing. Liquid-liquid interactions are governed by a tendency to reduce the energy of the system, which results in the separation of molecules that do not have affinity (i.e., oil and water) and a reduction of total surface area. For a given volume of liquid, spheres have the smallest surface area. Hence, when discrete packets form, they tend toward spherical volumes or droplets.
As predicted by the Rayleigh-Plateau instability, the protein TPX2 formed periodic droplets along microtubules during cell division (Figure 1). Moreover, the formation of droplets created discrete spaces for cofactors, the other molecules required for microtubule growth. Therefore, there were a finite number of spots where cofactors could co-localize, or group together. By discretizing the co-localization process, the TPX2 droplet formation can expedite microtubule growth. This is because it simplifies a process that would have otherwise been chaotic. Imagine you are at a concert, trying to find your friends: it would be easier to find them in discrete seating rather than in the standing room (mosh pit). In the same way, the Rayleigh-Plateau instability predicts the formation of droplets that maintain order in microtubule assembly. Thus, droplet formation leads to faster, precise, and efficient cellular division. In conclusion, Setru and Gouveia’s work demonstrated that a physical law governing liquid-liquid interactions can explain how the protein TPX2 facilitates fast, efficient microtubule branching (Setru et al., 2020). The model predicts that when TPX2 forms droplets along microtubules, it also promotes co-localization of other needed molecules, making the process more efficient. This exciting study highlights how physical laws can create a level of order in highly complex, vital biological processes.
Sagar Setru (co-first author of the paper) finds it "interesting when 'macro-scale' physics, the type of physics that we routinely observe with our eyes, matches nanoscale physics. It's even crazier that we find that this nanoscale physics plays a crucial role in a biochemical system. Such systems tend to be much more difficult to work with quantitatively compared to non-biological systems, which is why describing them in terms of physics has been uncommon, though that is changing as technology improves. I think that there are bound to be more examples where the physics of fluids can be brought to bear on molecular biology.”
Likewise, Bernardo Gouveia (co-first author) was "surprised to find that the everyday physics of how a faucet stream breaks up into droplets plays a role in establishing order in a nanoscale biochemical process. There are also many areas of cell biology where filaments (like microtubules) and liquid-like condensed proteins (like TPX2) come together, so it will be interesting future work to see if there are more examples of surface tension physics that play a role in molecular biology.”
The original article was published in arXiv on September 3, 2020. Please follow this link to view the full version. A video abstract of the paper, created by the authors, can also be viewed here.
References
King, Matthew R., and Sabine Petry. 2020. “Phase Separation of TPX2 Enhances and Spatially Coordinates Microtubule Nucleation.” Nature Communications 11 (1): 270. https://doi.org/10.1038/s41467-019-14087-0.
Petry, Sabine. 2016. “Mechanisms of Mitotic Spindle Assembly.” Annual Review of Biochemistry 85 (1): 659–83. https://doi.org/10.1146/annurev-biochem-060815-014528.
Setru, Sagar U., Bernardo Gouveia, Raymundo Alfaro-Aco, Joshua W. Shaevitz, Howard A. Stone, and Sabine Petry. 2020. “A Hydrodynamic Instability Drives Protein Droplet Formation on Microtubules to Nucleate Branches.” ArXiv:2001.06389 [Cond-Mat, Physics:Physics, q-Bio], February. http://arxiv.org/abs/2001.06389.
Walczak, Claire E., and Rebecca Heald. 2008. “Chapter Three - Mechanisms of Mitotic Spindle Assembly and Function.” In International Review of Cytology, edited by Kwang W. Jeon, 265:111–58. A Survey of Cell Biology. Academic Press. https://doi.org/10.1016/S0074-7696(07)65003-7.
Zhivotovsky, B., and S. Orrenius. 2010. “Cell Cycle and Cell Death in Disease: Past, Present and Future.” Journal of Internal Medicine 268 (5): 395–409. https://doi.org/10.1111/j.1365-2796.2010.02282.x.