Written by Ashley Chang (MOL, 2021) and Rebekah Rashford (PNI, G3)
Physiological decline is a natural component of human aging. One of the biological processes perhaps most rapidly affected by this decline is that of reproduction in women. The quantity and quality of a woman’s eggs decreases as she ages, thereby reducing the likelihood of a successful pregnancy as she approaches her late 30s to early 40s. Pregnancy in humans at all is relatively impossible after menopause, which typically occurs in the late 40s and beyond. Because of these biological restrictions, doctors and researchers have developed treatments to help women who want to have children later in life, such as freezing their eggs or in vitro fertilization followed by freezing of the embryos. While these treatments have undoubtedly changed the landscape of modern conception and fertility, they do not directly combat the deleterious effects of reproductive aging. Instead of creating systems that circumvent the inevitable, what if we could challenge the issue head-on by preventing deterioration in the quality of the egg precursor, the oocyte, and extending the reproductive age-span?
Princeton University Professor Dr. Coleen Murphy and her lab aim to understand the mechanisms regulating reproductive decline, with the larger goal of discovering the processes governing longevity as a whole. They use Caenorhabditis elegans (C. elegans), a roundworm, as a model organism for their work because like humans, the C. elegans reproductive span lasts only for one-third to one-half of their entire life span. Additionally, the underlying cause for their reproductive decline is that of deteriorating oocytes—similar to humans.
A recent paper led by postdoctoral scholar Dr. Nicole Templeman investigates how highly conserved signaling pathways involved in growth, development, and cell maintenance regulate the rate of age-related reproductive decline. These processes are studied because of their association with increasing infertility, miscarriage, and birth defects.
In this article, Templeman et al. start by reporting two previous discoveries in C. elegans that provide the basis for their proposed control mechanism for reproductive aging. The first is that of a signaling pathway involved in growth and development, the Small body/Male tail abnormal (Sma/Mab) branch of the Transforming growth factor 𝛽 (TGF-𝛽) signaling network. In previous studies by the Murphy lab, loss of this pathway’s activity, specifically in a layer of epithelial tissue called the hypodermis, was found to slow reproductive aging without having a pronounced effect on lifespan. By acting interdependently with other growth and developmental processes in the hypodermis, the TGF-𝛽 Sma/Mab signaling pathway results in a cascade of events that eventually reaches the reproductive system to modulate its aging processes. The second previous discovery discussed was the loss of a transcription factor, cAMP response element-binding protein (CREB), that contributes to metabolic mechanisms of reproductive aging. The Murphy lab found that without this transcription factor, reproductive span increases without simultaneously prolonging lifespan. It was with this prior knowledge that the authors hypothesized that these two components responsible for C. elegans growth and metabolism, TGF-𝛽 Sma/Mab and CREB, may also control reproductive decline independently from overall longevity, which is important considering that there is research demonstrating a relationship between the two types of aging. Templeman et al. aimed to elucidate how exactly TGF-𝛽 Sma/Mab signaling and CREB activity communicate with each other and with germline tissue (sex cells) to induce reproductive changes.
The authors first reproduced their previous results demonstrating that loss of crh-1, the gene encoding CREB, leads to an extended reproductive span and higher quality of aging oocytes without affecting life expectancy. Their previous studies had shown that crh-1 and TGF-𝛽 Sma/Mab loss-of-function mutants have similar transcriptional profiles, meaning that they have comparable gene expression. The knowledge then that TGF-𝛽 Sma/Mab signaling specifically in the hypodermis governs reproductive aging suggested that the hypodermis may also mediate CREB’s control over reproductive decline. Templeman et al. tested this by rescuing crh-1 expression only in the hypodermis of crh-1(-) mutants (loss of a particular gene function is from here on indicated by (-) following the gene name). They found that adding in the missing CREB from the crh-1(-) mutants only in the hypodermis reversed their extended reproductive span as well as their improved aging oocyte quality. This provided support for their hypothesis that the loss of CREB signaling specifically in the hypodermis is beneficial in delaying age-dependent reproductive decline and that this process occurs separately from other regular tissue aging.
The next step was to understand if and how CREB and TGF-𝛽 Sma/Mab signaling interact to regulate the rate of reproductive aging. The research team first determined whether CREB and TGF-𝛽 Sma/Mab work in parallel, or if one regulates the other. Using sma-3, which encodes the SMA-3 signaling protein, as a marker for the TGF-𝛽 Sma/Mab pathway, and crh-1 as a marker for CREB functionality, they performed two experiments in which they added either SMA-3 or CREB back to animals that lacked both. The authors found that rescuing hypodermal sma-3 in crh-1(-);sma-3(-) mutants was not sufficient to reduce their extended reproductive span. This indicated that CREB was necessary for hypodermal TGF-𝛽 Sma/Mab signaling to change the rate of reproductive decline, but it was still unclear if the pathways acted in series. To test this, they restored hypodermal crh-1 expression in those same crh-1(-);sma-3(-) mutants. This rescue was sufficient to shorten the mutants’ extended reproductive span, indicating that CREB indeed acts downstream of TGF-𝛽 Sma/Mab. This is because even without SMA-3, the restoration of CREB allowed for continuation of the pathway. These findings suggested that decreased TGF-𝛽 Sma/Mab signaling leads to reduced CREB activity in the hypodermis, which in turn is responsible for slowing reproductive aging.
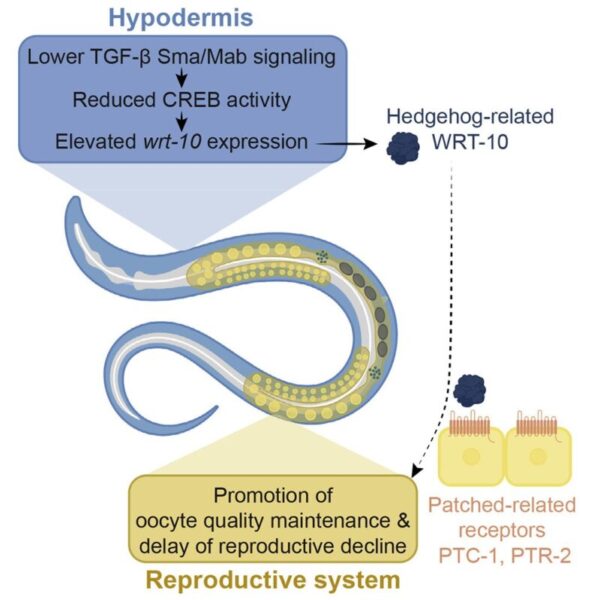
Although the authors determined the processes at work in this phenomenon, and even how they interacted, a gap still remained between hypodermal CREB activity and germline consequences. To understand CREB’s functions, they examined genes whose expression was altered (either decreased or increased) as a result of CREB loss. Templeman et al. found that wrt-10, which encodes the Hedgehog-related signaling factor WRT-10, had increased expression in the absence of CREB. This was particularly interesting because Hedgehog signaling molecules in fruit flies can serve metabolic roles in addition to their typical embryonic cell differentiation responsibilities (Hedgehog homologs in other organisms, such as C. elegans, are considered Hedgehog-related), which would explain WRT-10’s relevance to CREB, another metabolically-related protein. Elevated WRT-10 allowed for increased late-mating capacity, meaning that successful offspring delivery by older animals was more likely, and its upregulation in crh-1(-) mutants meant that CREB controlled its transcription. Hypodermal CREB activity limits WRT-10 expression, so in the absence of the protein, more WRT-10 is available to slow reproductive aging. Knockdown of wrt-10 impaired the ability of aged mutants to produce progeny and reduced oocyte quality, while hypodermal overexpression of wrt-10 in wild-type animals extended the reproductive span and improved quality of aging oocytes. The authors then hypothesized that WRT-10 serves as the signaling molecule that links the hypodermis and the reproductive system.
To determine the mechanism of WRT-10 signaling, the authors posited germline Patched-related receptors as likely receivers of the Hedgehog-related signaling factor, since the Patched receptor in fruit flies is crucial to Hedgehog signaling. When the authors generated worms with germline-specific knockdown of genes that encoded Patched-related receptors PTC-1 and PTC-2, they found a significant decline in late-mating capacity and increased age-dependent oocyte deterioration in animals overexpressing hypodermal wrt-10. These findings suggested that WRT-10 derived from the hypodermis signals via PTC-1 and PTC-2 in the germline to delay reproductive aging.
This study presents a notable model that links metabolic processes with reproductive decline through a hypodermis-to-germline signaling axis (Figure 1). Lower TGF-𝛽 Sma/Mab signaling in the hypodermis decreases CREB activity in the tissue, which then elevates WRT-10. This signaling molecule travels to the germline, where it comes in contact with PTC-1 and PTC-2 receptors, an interaction that promotes the delay of reproductive aging. The key takeaway is this: CREB-dependent transcription is the critical connection between two major pathways, TGF-𝛽 Sma/Mab and Hedgehog-related/Patched-related signaling, and facilitates tissue-to-tissue communication in regulating the rate of age-dependent reproductive decline.
Templeman et al.’s work makes room for further discussion on growth- and metabolism-mediated effects on aging oocyte maintenance and reproductive span. While these are preliminary results, continuing research in this area of study will help us better understand the molecular mechanisms underlying aging processes and eventually how reproductive timelines may potentially be altered.
This original article was published in Developmental Cell on July 6, 2020. Please follow this link to view the full version.
Figure used from Templeman et al., (2020).