Review by Abigail Stanton (MOL, G3)
At first glance, Caenorhabditis elegans might not look like much. Measuring in at about 1 mm, these laboratory worms have much simpler biology than your average human. However, this simplicity makes them convenient subjects to study the most basic functions of life because their entire lives from birth to reproduction to death play out over the course of weeks instead of decades. The Murphy Lab in the Department of Molecular Biology at Princeton uses these worms to ask questions about the aging process: what happens at a molecular level that causes us to age, and how can we promote longer, healthier lives?
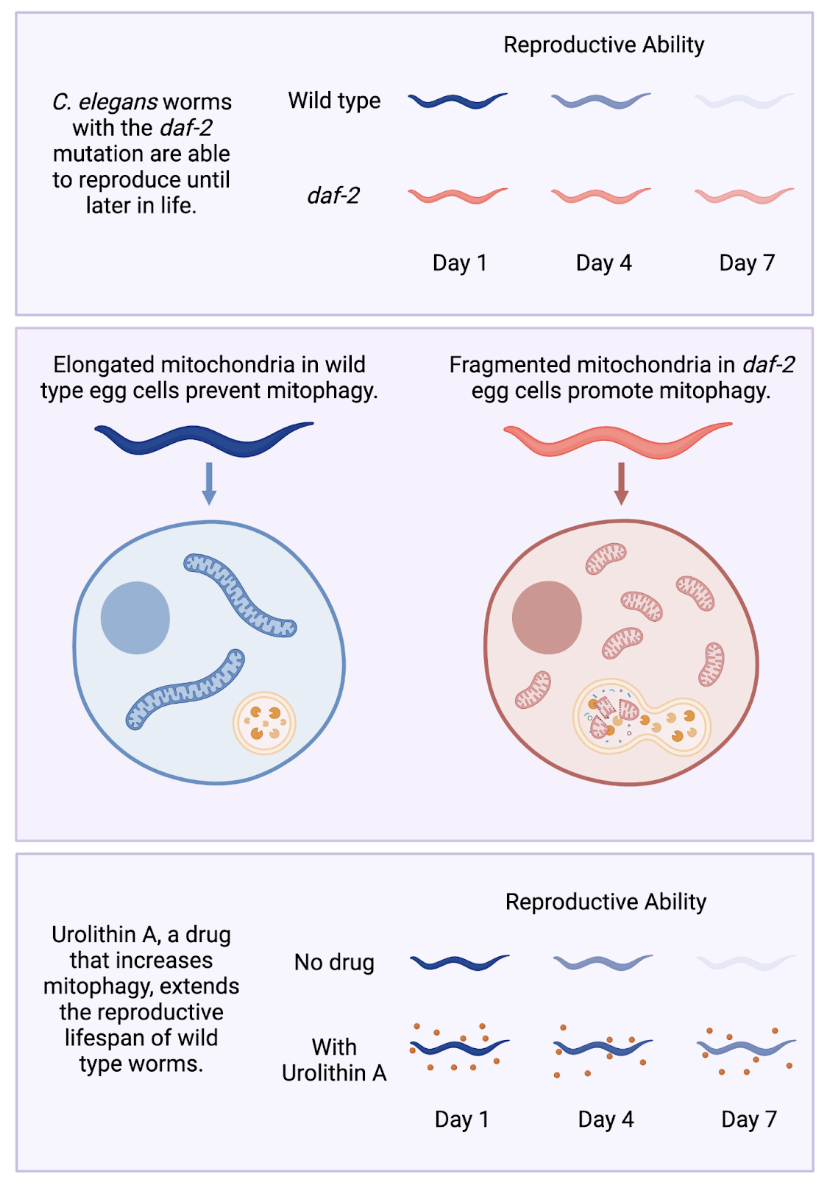
In a recent study from the Murphy lab, a team of researchers led by graduate student Vanessa Cota focused on one particular aspect of aging: reproductive decline. They started with the observation from previous research that worms with a mutation in a signaling protein called daf-2 not only lived longer, but were also able to reproduce later in life than typical (wild type) worms, likely due to maintenance of high quality eggs. Setting out to understand how these worms maintained high-quality eggs for longer, Cota and colleagues discovered the molecular mechanisms behind this extended reproductive longevity and showed that an existing drug, known to be safe for use by humans, could extend the reproductive lifespan of worms. This discovery has promise for therapeutic applications in humans, an exciting prospect as fertility issues pose a major challenge for would-be parents who wait until later in life to have children.
Mutant worms have high-quality eggs with a “low-quality” trait
Mitochondria are small structures within cells that generate energy for the cell. While often depicted as small jelly bean shapes, mitochondria often fuse together to form an elongated network capable of producing energy more efficiently than the fragmented “jelly beans” alone. In cells that make up the muscles and neurons of C. elegans worms, these mitochondrial networks become more fragmented with age. However, muscle and neural cells from daf-2 mutant worms which live longer than typical worms maintained an extended network of mitochondria as they aged. This led researchers to consider elongated mitochondria to be a sign of high-quality, healthy cells. On the other hand, small and fragmented mitochondria signal low cell quality and declining health.
This evidence from the literature led Cota and colleagues to hypothesize that egg cells of the daf-2 mutant worms, like muscle and neural cells, should maintain this elongated mitochondrial network, while egg cells from typical worms should lose it more quickly as their reproductive health declines. However, the researchers were surprised to see that the reverse was true: egg cells from typical worms maintained “high-quality,” elongated mitochondria into old age, even after they had lost the ability to reproduce. Conversely, mitochondria in the egg cells of daf-2 mutant worms were small and fragmented–not just in old age but throughout their lifespan. With this observation, the team had to revise their view: unlike in other types of cells, fragmented mitochondria in egg cells corresponded to a healthier state.
Breaking mitochondria apart isn’t enough to make healthy eggs
To test if fragmented mitochondria in egg cells were needed to extend the reproductive lifespan of the worms, Cota et al. turned to the proteins that directly cause mitochondria to fuse together or break apart: FZO-1 and DRP-1. If fragmented mitochondria are an essential part of what makes the eggs of the mutant worms healthy, then blocking mitochondrial fragmentation to keep them fused together should decrease the health of these mutant egg cells. Indeed, this is what they saw. The daf-2 mutant worms that had intact mitochondria networks weren’t able to reproduce as late in life as wild type worms.
Given these findings, Cota et al. attempted the opposite experiment: if they promoted fragmented mitochondria in wild type egg cells, could they increase reproductive lifespan? Unlike before, the results were not entirely expected. Blocking mitochondrial fusion in wild type worms resulted in fragmented mitochondria like in the daf-2 worms, but it didn’t give them any reproductive benefits. In fact, these worms had a shorter reproductive lifespan compared to completely unaltered worms. Therefore, mitochondria fragmentation is necessary for the longer reproductive lifespan seen in daf-2 mutants but not sufficient to promote it in wild type worms. The daf-2 mutation must be doing something more than just causing mitochondria to break apart, but what?
Destroying damaged mitochondria is key for an extended reproductive lifespan
While fusing together allows mitochondria to produce energy more efficiently, fragmentation also serves an important functional purpose. If a mitochondrion is defective, it may no longer produce energy; or, even worse, it may cause damage to the cell–like a power plant leaking toxic waste. For this reason, defective mitochondria must be regularly separated from the rest of the extended network and destroyed by a process called mitophagy. Cota and colleagues hypothesized that the fragmented mitochondria of the daf-2 worm egg cells may be a sign that they are taking out the mitochondrial “trash” more frequently.
PINK-1 is one the major proteins involved in promoting mitophagy. As predicted by this new hypothesis, when PINK-1 was inactivated (leading to defective mitochondria sticking around for longer), daf-2 worms had shorter reproductive lifespans. These results indicated that mitophagy is necessary for extended reproduction. Unlike with mitochondrial fragmentation alone, these findings also carried over to worms without the daf-2 mutation. The researchers treated wild type worms with a drug called Urolithin A, which promotes mitophagy by causing PINK-1 to accumulate. These treated worms had an extended reproductive lifespan compared to wild type worms without the drug. Urolithin A treatment even worked on “middle-aged” worms that had begun to experience reproductive decline, making this a promising avenue to explore for human therapeutics.
This study demonstrates the value of following unexpected results where they lead. From the surprising observation that healthy eggs had a trait that previous studies would have labeled as “low-quality”, Cota and colleagues in the Murphy Lab tracked down the root cause of what makes those eggs healthier in the first place. Using this information, they found a way to make average eggs more healthy in the process. While more research is needed to determine if Urolithin A will work to extend human reproductive longevity like it does in worms, it has already been shown to be safe for human use and effective in promoting mitophagy in other tissues. Therefore, this therapeutic, already cheap and readily available, could be adopted very soon as an intervention to prevent reproductive decline.
The original article discussed here was published in PLOS Genetics on September 20th, 2022. Please follow this link to view the full version.
